Classic Magnetic Beads-Based SELEX (MB-SELEX)
Classic magnetic beads-based SELEX (MB-SELEX) is an advanced technique used to select aptamers, which are short, single-stranded DNA or RNA molecules that can bind to specific target molecules with high affinity and specificity. The key feature of MB-SELEX is the use of magnetic beads functionalized with target molecules as a solid support matrix. This allows for efficient separation and manipulation of nucleic acid ligands during the SELEX process.
MB-SELEX is applicable to most of target molecules like proteins, whole cells, microbes, small molecules, etc., which can be bound and immobilized to the magnetic bead.
Fusion BioLabs further develop the main process of classic magnetic beads-based SELEX (MB-SELEX) screening methods to efficiently screen aptamers against small to large molecular targets. Following is the MB-SELEX process.
Main Steps of Classic Magnetic Beads-Based SELEX (MB-SELEX)
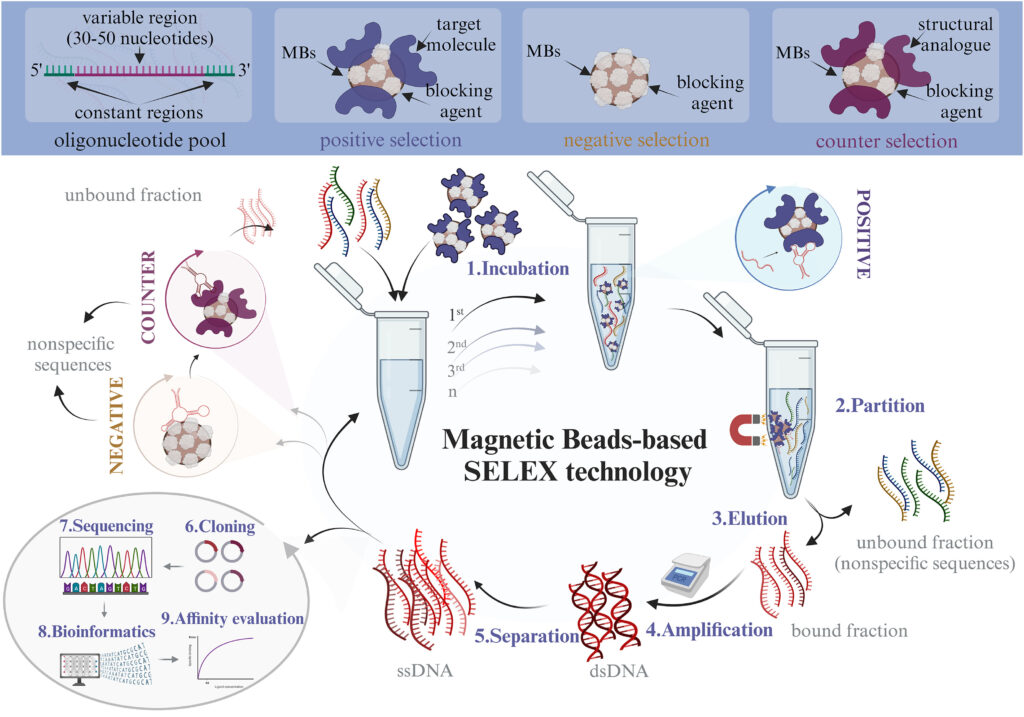
- Library Preparation: Begin by synthesizing a diverse library of single-stranded DNA (ssDNA) or RNA molecules.
- Target Immobilization: Functionalize magnetic beads with the target molecule (e.g., a protein, small molecule, or ion) that the aptamer will bind to.
- Incubation: Incubate the ssDNA/RNA library with the magnetic beads under conditions that favor binding of the nucleic acids to the target molecules.
- Separation: Use an external magnetic field to separate the magnetic beads (with bound nucleic acids) from the unbound nucleic acids in the solution.
- Washing: Wash the beads multiple times to remove non-specifically bound nucleic acids.
- Elution: Elute the bound nucleic acids from the beads, typically by using a buffer that disrupts the interaction between the target and the nucleic acids.
- Amplification: Amplify the eluted nucleic acids using polymerase chain reaction (PCR) for DNA or reverse transcription PCR (RT-PCR) for RNA.
- Renaturation: Denature the amplified nucleic acids to single strands to prepare for the next round of selection.
- Repetition: Repeat the incubation, separation, washing, elution, and amplification steps for several rounds (typically 8-15) to enrich for high-affinity aptamers.
- Final Analysis: After the final round, sequence the enriched aptamers and evaluate their binding affinity and specificity to the target molecule.
This iterative process ensures that the selected aptamers have high specificity and affinity for the target molecule.
Advantages of Classic Magnetic Beads-Based SELEX (MB-SELEX)
- Efficient Separation: Magnetic beads allow for quick and efficient separation of bound from unbound nucleic acids using a magnetic field, reducing the time and effort required for each selection round.
- Reduced Background Noise: The use of magnetic beads helps minimize nonspecific binding and background noise, increasing the likelihood of isolating high-affinity aptamers.
- Versatility: This method is versatile and can be applied to a wide range of targets, including proteins, small molecules, and even whole cells.
- Scalability: MB-SELEX can be easily scaled up for high-throughput applications, making it suitable for large-scale aptamer discovery projects.
- High Affinity and Specificity: The iterative selection process enriches for aptamers with high affinity and specificity for the target molecule, improving the quality of the final aptamer pool.
- Sample Handling: Magnetic beads can handle small sample volumes and are compatible with various buffer conditions, providing flexibility in experimental design.
So, MB-SELEX combines efficiency, precision, and versatility in a powerful package for aptamer selection.
Aptamer Development through MB-SELEX
Phase I: Library Enrichment via MB-SELEX
Iterative rounds (10-15 rounds) of screening an initial random library against a target for positive selection, and negative and/or counter-selection screening against sample matrix or counter-target(s) for specificity.
Criteria: Enrichment 8-30%.
Deliverables: full enrichment progress report
Timeline: 2-3 weeks
Phase II: Sanger Sequencing & Bioinformatic Analysis
Last round of positive binding candidates cloning into sequencing vector for polyclonal preparation and picking up to 48 clones (Basic Package and Standard Package) for Sanger sequencing.
Deliverables: Aptamer candidate sequence information, sequence alignment, family cohort, secondary structure analysis, docking simulation et al.
Timeline: 1-2 weeks
Phase III: NGS Sequencing & Bioinformatic Analysis (optional)
Next-Generation Sequencing (NGS) of last positive round and representative rounds, negative binding rounds, and control of initial non-enriched library. Bioinformatically analyzing to identify the potential top aptamer candidates by ranking the aptamers based on their enrichment ratio or fold-change between positive and control/negative rounds.
Deliverables: NGS analysis full report
Timeline: 2-3 weeks
Phase IV: Aptamer Candidate Synthesis, Affinity Determination and Specificity Assessment
Depending on the results of data analysis, up to 96 aptamer hits (Premium Package or Premium plus Package) showing high enrichment profiles and different structural families will be synthesized in small-scale to determine top candidates for further characterization. Top aptamer candidates will have a high enrichment ratio and high statistical significance in the positive rounds compared to control.
Final ranking and validation to confirm their binding affinity and specificity: each top aptamer candidate (up to 8) will be synthesized and quantitatively tested for KD affinities via GO-SELX, Capture-SELEX, or AuNP-SELEX, biolayer interferometry (BLITz), surface plasmon resonance (SPR), enzyme-linked aptamer-sorbent assay (ELASA) or gel shift assays.
Deliverables: 10 nmol each aptamer candidates, and full assay report showing KD value and specificity for each aptamer candidates.
Timeline: 3-4 weeks
Phase V: Aptamer Optimization & Assay Development
Depends on the criteria set by clients, the cost and timeline will be different.
Deliverables: Full assay development report and 10m nmol optimized aptamers.
Timeline: 2-4 weeks
Fusion Biolabs offer the following packages
| Basic Package | Standard Package | Premium Package | Premium plus Package | |
|---|---|---|---|---|
| Phase I: Library Enrichment via classic MB-SELEX | ||||
| Phase II: Sanger Sequencing & Bioinformatic Analysis | ||||
| Phase III: NGS Sequencing & Bioinformatic Analysis | ||||
| Phase IV: Aptamer Candidate Synthesis, Affinity Determination and Specificity Assessment | ||||
| Phase V: Aptamer Optimization & Assay Development | ||||
| Package Cost* | inquiry | inquiry | inquiry | inquiry |
1) Fusion BioLabs guarantees the services.
2) Fusion BioLabs owns its proprietary processes of aptamer development. However, the client owns full rights to the aptamers Fusion BioLabs developed for the client.
3) For multiple targets aptamer development, please contact us for special pricing.
References
Manea I, Casian M, Hosu-Stancioiu O, de-Los-Santos-Álvarez N, Lobo-Castañón MJ, Cristea C. A review on magnetic beads-based SELEX technologies: Applications from small to large target molecules. Anal Chim Acta. 2024, 1297:342325.
