The remarkable therapeutic efficacy of CD19 targeted CAR-T cells for hematological malignancies such as B cell acute lymphoblastic leukemia (ALL), non-hodgkin’s lymphoma (NHL) and diffuse large B-cell lymphoma (DLBCL) has led to the approval of five CAR-T cell products by the US food and drug administration (FDA). Thus, it has generated the impetus to broaden CAR-T cell therapy applications, resulting in growing production demand. Successful manufacturing is not only the foundation for every CAR-T cell clinical trial, but the choice of CAR-T cell manufacturing strategy and methodology also contributes to the phenotype and efficacy of the cells and drives the cost of goods.
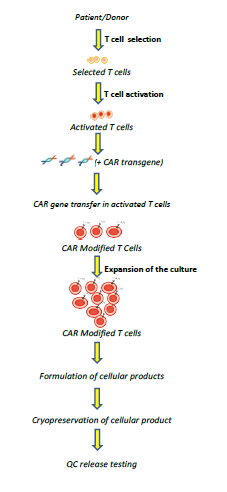
Despite the availability of multiple methodologies and cell manufacturing platforms, the core components of the CAR-T cell manufacturing procedure remain consistent between processes. They include source material collection, T cell isolation, activation, genetic modification, expansion, end of process formulation and cryopreservation. The stepwise CAR-T cell manufacturing procedure is described and illustrated below.
T Cell Activation
T Cell activation is a key step for preparing T cells for gene transfer and expansion. Various in vitro T cell activation methods have been established and they can be largely classified as T cell stimulation by either soluble antibodies, Expamer, antibody-coated beads, or artificial antigen presenting cells (AAPCs).
Transfer CARs into T Cells
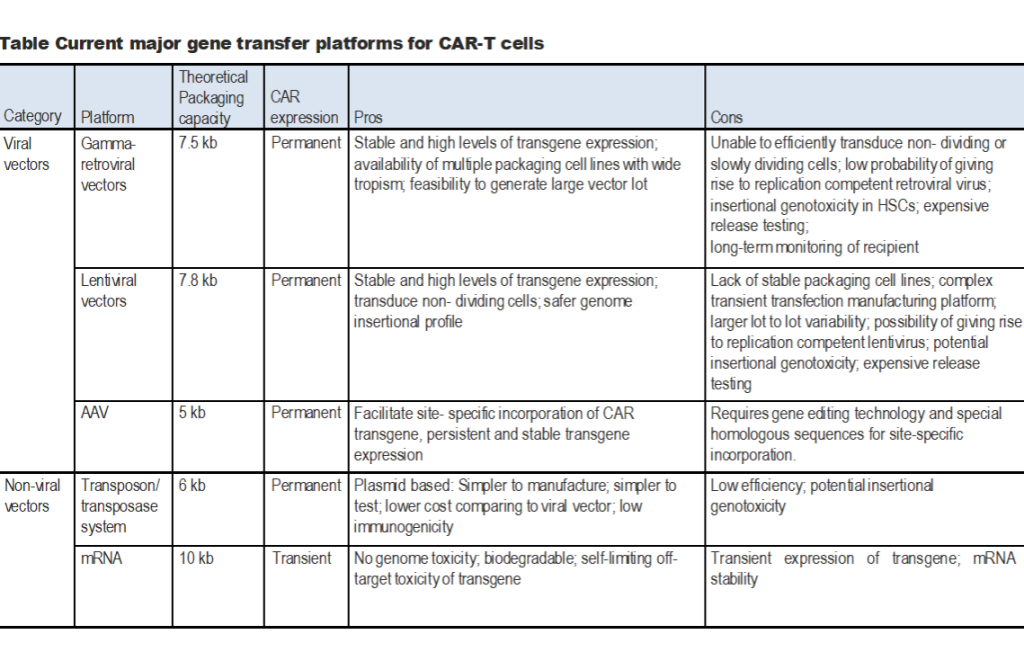
CARs can be introduced into T cells either permanently or transiently by using viral or non-viral gene transfer methodologies. Gammaretroviral vectors, lentiviral vectors and transposon/transposases are the three major approaches for permanently incorporating CAR transgenes into the genome. Alternatively, AAV-CAR (Adeno-Associated Virus-CAR) expression cassettes flanked by homology arms are also used in conjunction with CRISPR/Cas gene editing tools for site-specific CAR integration.
Fusion Biolabs provide four platforms to manufacture CAR-T cells
1. Generation of Chimeric Antigen Receptor T Cells (CAR-T cells) Using Gamma-retroviral Vectors
Transient transfection of 293T cells will be used for gammaretroviral particles production in culture media. The viral vector-containing supernatant is then filtered and used to infect T cells previously activated by soluble monoclonal antibodies (mAbs). Upon viral entry into T cells, the CAR transgene is integrated into the cell genome to ensure stable long-term expression.
2. Generation of Chimeric Antigen Receptor T Cells (CAR-T cells) Using Lentiviral Vectors
One of the most versatile gene transfer methods involves the use of recombinant lentiviral vectors since they can transduce both dividing and nondividing cells, are considered to be safe and provide long-term transgene expression since the integrated viral genome, the provirus, is passed on to daughter cells. Fusion Biolabs’ scientists have established a simple and direct platform to production of high titer lentivirus and further for CAR-T Cells generation.
3. Generation of Chimeric Antigen Receptor T Cells (CAR-T cells) Using Adeno-Associated Virus
The popular CRISPR/Cas9 genome editing system has been modified for use with adeno-associated virus (AAV). Precise knock-in of CAR cassette into specific gene locus or integration site include TRAC, TRBC to disrupt endogenous TCRs, and the PDCD1, CTLA-4 and other inhibitory checkpoint genes, etc. to enhance T cell functionality and prevent T cell exhaustion. Precise knock-in can lower the risks caused by random integration, as well as enhance the stability and function of the modified CAR-T cells. CRISPR–Cpf1/RNA-guided nuclease shows higher homology-directed repair (HDR) rate compared to Cas9 due to its unique biochemical characteristics. Fusion Biolabs’ scientists have developed platforms combining electroporation and adeno-associated virus (AAV) infection to deliver CRISPR/Cpf1 components and a HDR template into T cells, thus precisely integrate CAR sequence at a specific gene locus with high efficiency.
4. Generation of Chimeric Antigen Receptor T Cells (CAR-T cells) Using Sleeping Beauty Transposon System
Sleeping Beauty transposon system, a nonnviral gene transfer methods, can stably integrate in the genome of target cells and can be delivered using straightforward methods like electroporation. Fusion Biolabs have developed a platform for T cell genetic modification using Sleeping Beauty transposon system and electroporation with the Lonza Nucleofector II device for the stable expression of CAR molecules in T lymphocytes.
Order Information
Customized CAR-T cell generation or virus production for CAR-T cell transduction
| Catalog | Description | Unit | Price |
|---|---|---|---|
| 63101 | Gammaretrovirus production or CAR-T cell generation using gammaretroviral vectors | 1 Project | Contact Us |
| 63102 | Lentivirus production or CAR-T cell generation using lentiviral vectors | 1 Project | Contact Us |
| 63103 | CAR-T cell generation using AAV vectors | 1 Project | Contact Us |
| 63104 | CAR-T cell generation using Sleeping Beauty transposon system | 1 Project | Contact Us |
For complex projects, please fill the form for a customized quotation and proposal!